Paracelsus, born way back in 1493 in Switzerland, is credited with being the first medical scientist (started the use of chemically based medications over herbal remedies) and is also the founder of toxicology.
One of his more famous quotes states “All things are poison and nothing is without poison; only the dose makes a thing not a poison” .
So if the dose makes the poison, at what dosage will a chemical prove fatal to humans?
Acute Toxicity
Historically the acute toxicity of a chemical has been expressed by its LD50 rating, which is the amount of poison (pure active constituent) required in one dose to kill 50% of a batch of test animals, normally rodents, i.e. The Lethal Dose 50%. The LD50 is expressed in milligrams of the active constituent per kilogram of body weight of the test animals and the poison may be administered “in vivo” (into the animal) either orally, dermally or by inhalation.
To encourage lethal dose data that provides a more accurate extrapolation to humans, some countries like the United States require a non-rodent species as well as a rodent species for LD50 determinations. There is also pressure from animal welfare groups to move away from in vivo testing towards in vitro testing (outside the animal), where just its cells or blood samples are used in the evaluations making it not fatal to the test animal.
The debate as to which is better is lengthy and complicated by the fact that acute responses to varying doses of chemical are often not linear and chemicals with relatively low acute toxicity can have serious longer term chronic effects. However, as crude as LD50 may be regarded by the purist, it is a useful guide to determine acute toxicity and is the technique currently favoured in chemical safety data sheets (section 11, Toxicology).
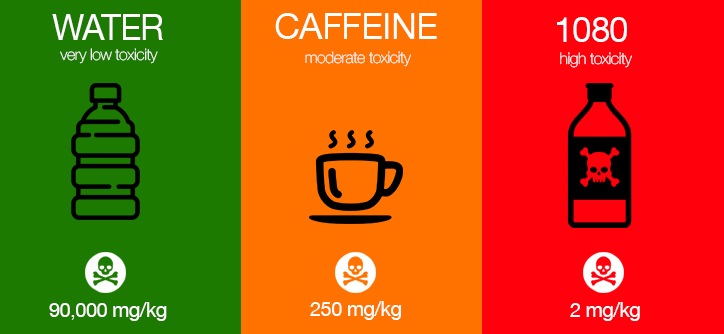
Looking at table 1 note that one of most acutely toxic substances known to man is botulinum toxin, where only 0.00085mg can kill half the population weighing 85 kgs, as opposed to caffeine whereby 79 cups of regular brewed coffee would need to be drunk one after the other to achieve the same result, as shown below.
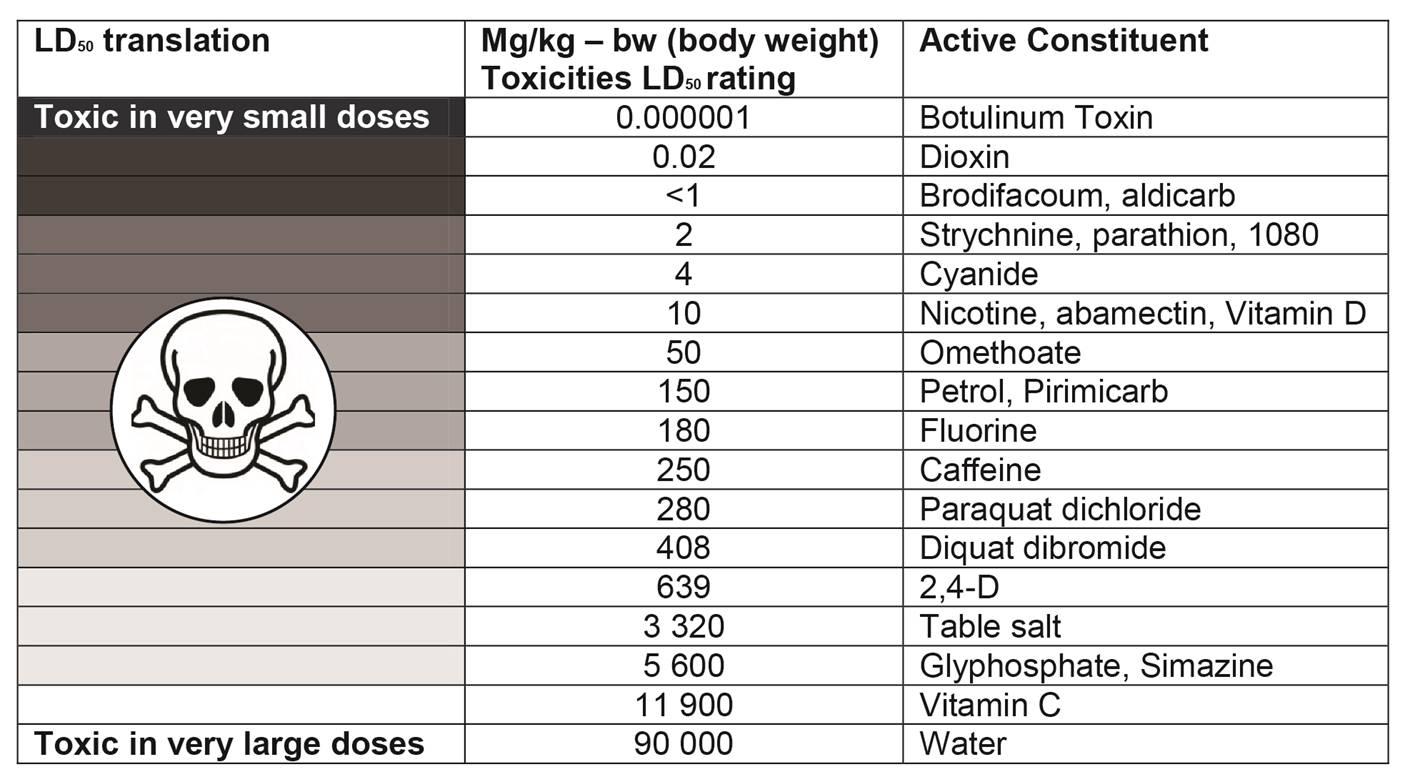 Table 1 – Comparative Acute Toxicities LD50
Table 1 – Comparative Acute Toxicities LD50
LD50 values are measured from zero up, therefore the lower the LD50 the more acutely toxic the chemical is and so an oral LD50 of 408 such as Diquat is much less toxic than a chemical with an LD50 of 4 such as cyanide. Milligrams per kilogram is the same ratio as parts per million and so it would require a dose of 4 parts cyanide for every million parts of body weight to be lethal to at least half of the test animals.
Poison Schedules
The SUSMP (Standard for the Uniform Scheduling of Medicines and Poisons) promotes uniformity of state/territory regulation throughout Australia regarding the:
• scheduling of poisons (10 schedules but only nine are pictured below in table 2);
• signal headings on labels for poisons;
• labelling and packaging requirements for poisons;
• additional controls on the availability and use of poisons.
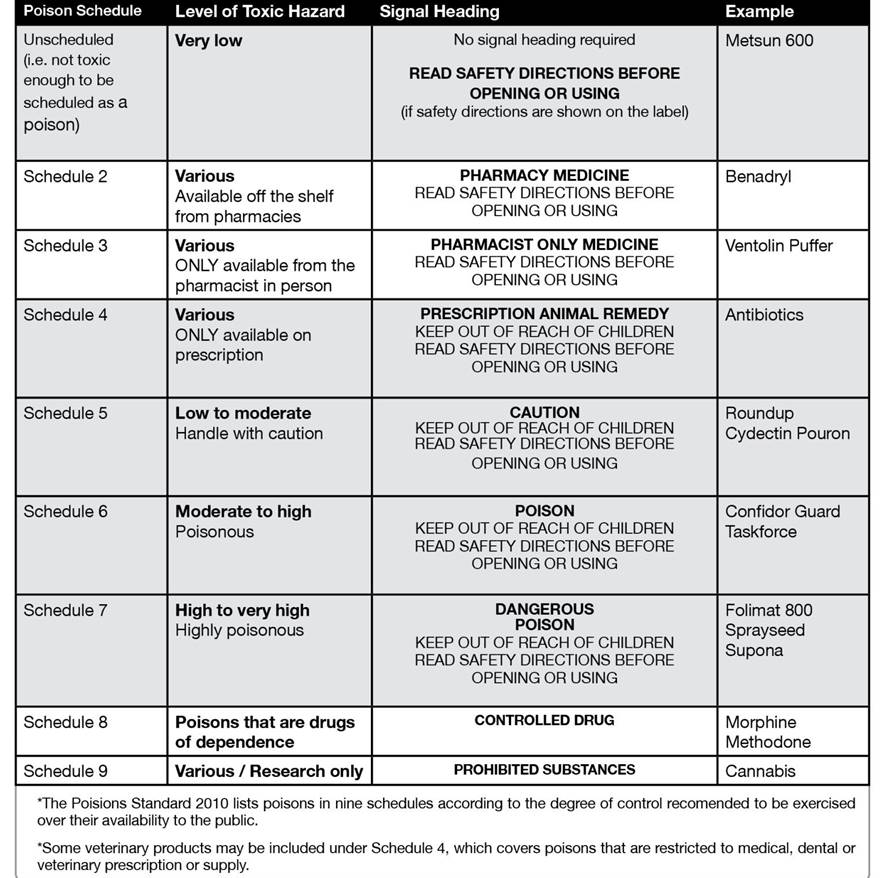 Table 2. The Poison Standard
Table 2. The Poison Standard
For agricultural and some domestic, medical and industrial poisons, Schedules 5, 6 and 7 represent increasingly stricter container and labelling requirements with special regulatory controls over the availability of the poisons listed in Schedule 7 poisons (1080, paraquat etc.). Products for domestic use must not include poisons listed in Schedule 7.
Roundup herbicide for instance is a Schedule 5 poison of low toxicity with the term “Caution” as its Signal Heading.
Poisons are not scheduled on a toxicity basis alone but also take into account many other criteria such as the purpose of use, potential for abuse, safety in use and the very need for the substance in the first place. Refer to www.tga.gov.au for further information.
Chronic Toxicity
Chronic toxicity is the adverse health effects from repeated exposures to a substance over a long time period (months or years) and often this is at low levels, such as hand spraying a pesticide at 1% concentration repeatedly each month for a year or years without appropriate personal protective equipment (PPE). This is in contrast to an acute toxicity event which could happen accidentally from a splash or a spill when mixing the pesticide in concentrated form.
Section 11 of a products safety data sheet will also detail any potential chronic effects from using a hazardous chemical (as well as the acute toxicity mentioned earlier) and general details if a product is carcinogenic (cancer causing), mutagenic (changes an organisms genetic information) and teratogenic (causes harm to the fetus or embryo during pregnancy).
Some chemicals that cause chronic health effects are still in use, like nicotine, diesel exhaust, formaldehyde to name a few. A more detailed list can be found here .
If you want to learn more about product labels, safety data sheets and how to safely handle, store and use chemicals, then come along to a Chemcert courseand discuss some of these issues with your peers and Chemcert trainer.
Icons in article banner sourced from Freepik