The quality of water being used in the spray tank to act as the carrier for your pesticides can have significant effects on how well those pesticides will work. It is surprising, however, to find that very few growers have had water quality tested.
Water with suspended materials such as clay, algae and other debris will block filters and possibly nozzles which makes spraying very frustrating. However, there are a range of water quality variables unseen to the naked eye that can affect specific pesticide formulations. The two that cause the most confusion are hardness and pH.
Water hardness
Water that is considered hard has high levels of calcium, magnesium or bicarbonate ions. Calcium and magnesium ions have positive electrical charges that enable them to bind with negatively charged products such as ‘weak acid’ herbicides in solution making them less soluble. Extreme cases can lead to the herbicide settling out in the spray tank or most commonly reducing the ability of the active ingredient to be absorbed through the plant leaf. Example of weak acid herbicides includes glyphosate and amine formulations of 2,4-D, MCPA, 2,4-DB, clopyralid and diflufenican.
Water hardness above 250 to 350 parts per million (ppm) (calcium carbonate – CaCO 3 equivalents) should be treated before adding weak acid herbicides.
The cations that can cause the most trouble for pesticides include:
- aluminium (Al +++ )
- iron (Fe +++ , Fe ++ )
- magnesium (Mg ++ )
- calcium (Ca ++ )
- sodium (Na + )
With magnesium and calcium being the most common cations causing water quality problems, aluminium can be a problem if alum (aluminium sulphate) has been used to remove (flocculate) clay and other particles from the spray water.
Bicarbonates can also affect herbicides such as Group A ‘dims’ (e.g. clethodim) and a 2,4-D amine at levels as low as 175 ppm. Bicarbonates are not detected by standard water hardness tests and must be tested for separately, to total hardness. Groundwater from areas with lots of limestone can be high in bicarbonates. Water high in bicarbonates can be overcome by substituting MCPA or a low volatile ester formulation of 2,4-D for the 2,4-D amine. Group A ‘dim’ herbicides can be helped with the use of ammonium sulphate.
To treat water for hardness and bicarbonates, use up to 1% crystalline ammonium sulphate or liquid formulations of ammonium sulphate such as a Liase®.
Water pH
The pH of a liquid is a method of describing how acid or alkaline the fluid is. A neutral pH is about 7 whereas a pH of 2 is very acidic and a pH of 14 is very alkaline. It is important to remember that the pH scale is a log scale. This means that a value of 6 is 10 times more acid than a pH of 7 while a pH of 8 is 10 times more alkaline than 7 and 100 times more alkaline than 6. The table below gives some examples of common materials and their pH.
Table 1. pH values of some common substances.
| pH | Substance |
| 14 | Sodium hydroxide (caustic soda) |
| 12.6 | Sodium hypochlorite (bleach) |
| 11.5 | Ammonia |
| 10.2 | Magnesium hydroxide (antacids) |
| 9.3 | Sodium borate (borax) |
| 8.4 | Sodium bicarbonate (baking soda) |
| 8.1 | Seawater |
| 7.4 | Human blood |
| 7.0 | De-ionised water |
| 6.8 | Tea |
| 6.7 | Milk |
| 6.0 | Rainwater |
| 4.5 | Tomatoes |
| 4.2 | Orange juice |
| 4.0 | Wine & beer |
| 2.8 | Vinegar |
| 2.2 | Lemon juice |
| 2.0 | Stomach acid |
| 1.0 | Battery acid |
| 0.0 | Hydrochloric acid |
There are many half-truths in the marketplace about the effect of pH on pesticides. It is well recognised that pH above 8 will reduce the life in the tank of certain pesticides such as organophosphate insecticides. Despite this, the effect of high pH on herbicides is mostly overstated.
Glyphosate has been found to work slightly better in moderately acid solutions, but this effect is from the precipitation of calcium compounds before forming calcium glyphosate on drying of the droplets on the leaf. Treat the water with ammonium sulphate before adding glyphosate, and you solve this issue.
If the pH of water used in the spray tank is between 6 and 8, it is suitable for spraying.
Acidic water (pH < 5) can affect the stability of mixes and lead to gelling of salt-based products and has been found to increase the volatility of herbicides such as dicamba.
Water above pH 8 can modify droplet contact on the leaf surface, reduce the stability of mixes and change product solubility. 
Figure 1 This grower was told to drop the pH of his spray. He added citric acid and added another three products. Source: R. Buttimor
So, do I need to reduce the pH of my water?
If your water is between a pH of 6 and 8, the answer is no.
Something that is rarely discussed is that the addition of the pesticide will modify the pH of the solution. Therefore, each pesticide user needs to test the water before the addition of pesticides and then check the pH after the addition of the pesticide. They will be very different.
The addition of glyphosate to the spray solution will drop the pH of the spray mix from 8 to less than 5. In the image below the test strip on the right is town water which usually has a pH of about 8.5, compared with the test strip to the left which is from a 1% glyphosate 450 solutions using town water, which is below a pH of 4. 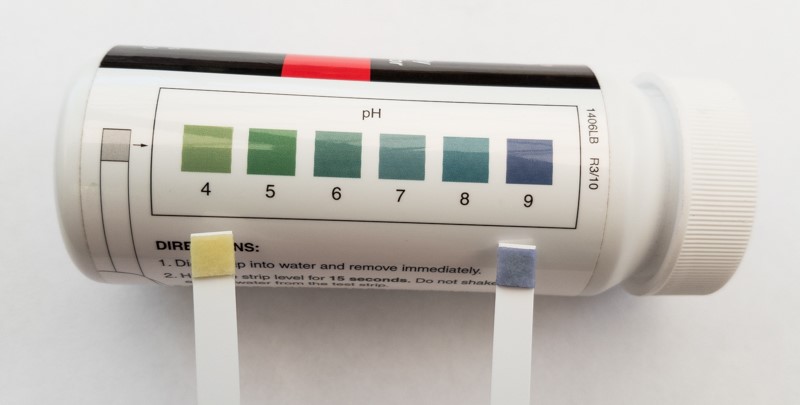
Figure 2 Adding glyphosate will drop the pH of the tank mix 2 to 3 units depending on initial pH, formulation and rate of glyphosate. The pH after the addition of glyphosate is less than 4 – the yellow test strip. Town water right. Source: AGRONOMO
Recent research in the United States has found drift damage from dicamba continued to be a problem despite the mandating of using XC and UC spray quality. They found one cause was the addition of glyphosate to the mix which reduced the pH of the spray solution (Table 2). The volatilisation of dicamba increases with decreasing pH. Different formulations of dicamba were found to drop the spray solution pH from 7.8 to between 6.5 and 6.9. However, the addition of different formulations of glyphosate all dropped the spray solution to 5 or lower.
Table 2 Effect of different formulations of dicamba and glyphosate on spray solution pH. Source: Larry Steckel
| Starting pH of water | Dicamba added (3 different formulations) | Glyphosate added (3 different formulations) |
| 7.8 | 6.9 | 4.8 |
| 6.5 | 4.8 | |
| 6.7 | 5.0 |
Currently, the recommendation for dicamba use over the top of dicamba-resistant crops is not to add glyphosate to the mix. This will minimise pH drop and therefore reduce the volatilisation of dicamba and off-target damage to sensitive crops and areas.
Water testing
Knowing the quality of the water you are using is essential for effective pesticide application. Water should be initially tested by a qualified laboratory to establish an accurate baseline for your water quality.
It is important to remember that water quality can vary over time depending on its source. Scheme or town water quality tends to change very little. However, water from surface sources such as dams, tanks and rivers will vary depending on rainfall and other factors. Groundwater can also vary over time depending on how much is being pumped and the recharge rates of the aquifer.
Water should be tested for:
- pH
- total hardness
- bicarbonate
- salinity (electrical conductivity) or total dissolved salts (TDS)
Test strips can be used to quickly check water quality before and after the addition of pesticides and monitor changes in water quality between laboratory tests.
Hand-held meters are available; however, these will need regular calibration to maintain accuracy.
Where can I get my water tested?
Check with your pesticide reseller or look for accredited laboratories in your state.
High-quality test strips can be purchased online from companies such as Hach .
Water testing kits for swimming pools will not be as accurate as those from a scientific supply company.
Further information
GRDC spray water quality
GRDC adjuvants – oils, surfactants and other additives for farm chemicals.




